Preparing for an influx of antibody testing? ZEUS SARS-CoV-2 Test Systems are the answer.
| COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). An interactive web-based dashboard to track COVID-19 in real time. "Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Inf Dis. 20(5):533-534. doi: 10.1016/S1473-3099(20)30120-1" |
Are you ready for increased antibody testing demand?
Do you know someone who is wondering if they had COVID-19 and were asymptomatic? Are they thinking “Did I have COVID-19 and not realize it, was that short term cough due to allergies or did I have mild symptoms for COVID-19?”
As COVID-19 cases surge (see Johns Hopkins COVID-19 tracker), more people are questioning if they have been exposed to the SARS-CoV-2 virus. SARS-CoV-2 antibody testing provides evidence of past exposure to SARS-CoV-2 and the presence of potentially life saving or, at minimum, protective antibodies. If you haven't seen an increase in requests for antibody testing, don't be surprised if you do soon.
Are you or your partner labs ready for an influx of new SARS-CoV-2 antibody testing requests?
If you haven't implemented serological antibody testing in your lab or if you are looking for a backup testing option to meet upcoming demand, consider ZEUS's SARS-CoV-2 antibody offerings.

With over 40 years of experience in the infectious disease space, you can count on quality products from Zeus Scientific. We know you will be satisfied, speak to your representative today to get a validation kit, and see for yourself!
What Makes ZEUS Unique? The Dual Antigen Difference: Specificity Matters
The ZEUS ELISA SARS-CoV-2 Test Systems detect antibodies to TWO SARS-CoV-2 antigens - the highly immunogenic N-protein and the neutralizing spike receptor binding domain (S1-RBD) - to provide superior sensitivity and specificity.
ZEUS ELISA SARS - CoV-2 Antibody Test Systems Provide
- Easy to use universal procedure for all kits
- Fast turnaround time for results
- Unique dual antigen approach
- Accurate results with high specificity and sensitivity
- Validated automation
Why wait? Order now and be prepared!

The Dual Antigen Difference: Specificity Matters
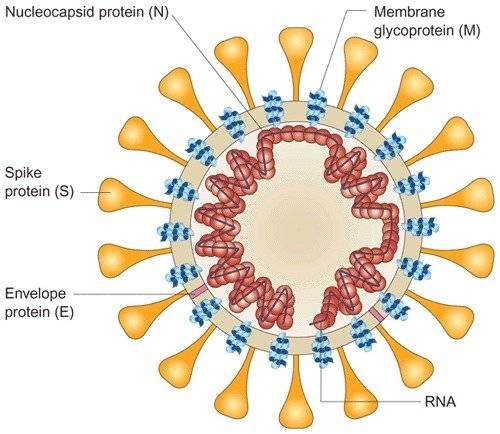 Schematic labelled diagram of Coronavirus (SARS-CoV)2 |
S1-RBD: The surface glycoprotein of the virus, termed the spike (S) protein, mediates attachment of the virus to human cells via its receptor‐binding domain (RBD)1 and mediates fusion of viral and cellular membranes. Antibodies binding to the spike protein, and especially to the RBD domain, can neutralize SARS-CoV-2. N-Protein: The nucleocapsid protein (N-protein) is the most abundant protein in coronavirus. The N-protein is a highly immunogenic phosphoprotein, and it is normally very conserved. The N protein of coronavirus is often used as a marker in diagnostic assays. |
During virion assembly, N protein binds to viral RNA and leads to formation of the helical nucleocapsid. The abundance and high hydrophilicity of N protein are supposed to contribute to potent immunity after coronavirus infection.
ZEUS’s dual antigen approach is unique as most available tests are limited to one or the other. By detecting antibodies directed against both the highly immunogenic N-protein and the neutralizing S1-RBD our assay is optimized for superior sensitivity and specificity.
Test with Confidence. Test with ZEUS.
Learn more at zeuscovid.com. We are here to help with your transition!
Sincerely,
The ZEUS Scientific Family
1 Wrapp et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 367, 1260-1263 (2020).
2 Taken from Peiris et al. Severe acute respiratory syndrome. Nature Medicine Vol 10 (12) (2004).
FDA EUA Disclaimer: This test has been authorized only for the presence of IgG antibodies against SARS-CoV-2, not for any other viruses or pathogens. This test has been authorized by FDA under an EUA for use by authorized laboratories. This test has not been FDA cleared or approved; This test is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner.
Back Share