ZEUS ELISA SARS-CoV-2 IgG Performance Summary
Authorized by FDA for Emergency Use. View the SARS-CoV-2 IgG EUA here.
| Clinical Performance Studies |
Two separate cohorts of clinically characterized specimens were tested:
- COVID-19 RT-PCR Positive Patient Specimens (clinical positive, n=35). The date between EUA-approved PCR test result and specimen draw was 3 to 37 days, with an average of 15.97 days and a median of 14 days.
- COVID-19 RT-PCR Negative and Pre-Pandemic Patient Specimens (clinical negative, n=214)
The ZEUS ELISA SARS-CoV-2 IgG Test System performed as follows: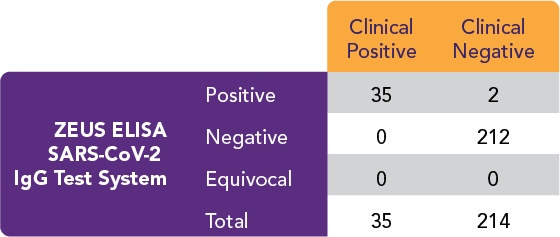
Positive Percent Agreement (PPA) = 35/35 = 100%
95% CI = 90% to 100%
Negative Percent Agreement (NPA) = 212/214 = 99.1%
95% CI = 96.6% to 99.7%
| Non-Clinical Performance Studies |
Specificity – Pre-pandemic healthy blood donors
Ninety normal healthy blood donors collected in the Northeastern US prior to November of 2019 were tested on the ZEUS ELISA SARS-CoV-2 IgG Test System. One specimen was equivocal and the remaining 89 specimens were negative.
The specificity was determined to be 89/90 = 98.9%
Specificity – Pre-pandemic patients with various respiratory illnesses
Ninety specimens were evaluated on the ZEUS ELISA SARS-CoV-2 IgG Test System that were collected from patients with a variety of respiratory illnesses. These specimens were tested for the following infectious agents; MERS, RSV, FluA, FluB, Parainfluenza, Adenovirus, Enterovirus, Mycoplasma pneumonia, Legionella, B. pertussis, and C. pneumonia. Many specimens were positive for antibody to multiple agents. One of the ninety specimens was positive and the remaining 89 specimens were negative.
The specificity in this cohort was determined to be 89/90 = 98.9%
FDA EUA Disclaimer: This test has been authorized only for the presence of IgG antibodies against SARS-CoV-2, not for any other viruses or pathogens. This test has been authorized by FDA under an EUA for use by authorized laboratories. This test has not been FDA cleared or approved; This test is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner.