ZEUS ELISA SARS-CoV-2 Total Antibody Performance Summary
Authorized by FDA for Emergency Use.
| Clinical Performance Studies |
Two separate cohorts of clinically characterized specimens were tested:
- COVID-19 RT-PCR Positive Patient Specimens (clinical positive, n=50). Fifty serum or plasma samples were obtained from donors that had previously tested positive via an EUA approved RT-PCR test system.
- COVID-19 RT-PCR Negative and Pre-Pandemic Patient Specimens (clinical negative, n=264) Eighty-four serum samples were obtained from donors that had previously tested negative via an EUA approved RT-PCR test system. In addition, 90 negative serum samples obtained from healthy donors and 90 negative serum samples obtained from febrile donors suspected of respiratory or other illnesses were collected prior to November of 2019.
The ZEUS ELISA SARS-CoV-2 Total Antibody Test System performed as follows: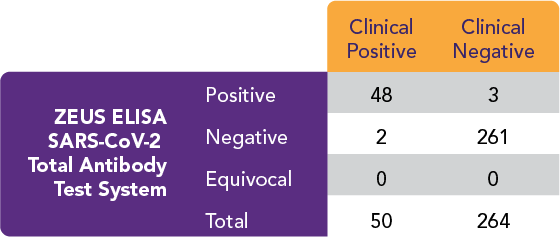
Positive Percent Agreement (PPA) = 48/50 = 96.0%
95% CI = 86.5% to 98.9%
Negative Percent Agreement (NPA) = 261/264 = 98.9%
95% CI = 96.7% to 99.6%
| Non-Clinical Performance Studies |
Specificity – Pre-pandemic healthy blood donors
Ninety normal healthy blood donors collected in the North Eastern US prior to November of 2019 were tested on the ZEUS ELISA SARS-CoV-2 Total Antibody Test System. All 90 specimens were negative.
The specificity was determined to be 90/90 = 100%
Specificity – Pre-pandemic patients with various respiratory illnesses
Ninety specimens were evaluated on the ZEUS ELISA SARS-CoV-2 Total Antibody Test System that were collected from patients with a variety of respiratory illnesses. These specimens were tested for the following infectious agents: MERS, RSV, FluA, FluB, Parainfluenza, Adenovirus, Enterovirus, Mycoplasma pneumonia, Legionella, B. pertussis, and C. pneumonia. Many specimens were positive for antibody to multiple agents. All ninety specimens were negative.
The specificity in this cohort was determined to be 90/90 = 100%