ZEUS ELISA SARS-CoV-2 Antibody Test Systems
Powered by full automation
Both the ZEUS SARS-CoV-2 IgG and Total Test Systems are authorized by the FDA for Emergency Use.
The Dual Antigen DifferenceDoes your antibody test detect antibodies to both the nucleocapsid protein and the S1-RBD (spike protein) of SARS-CoV-2? |
The ZEUS ELISA SARS-CoV-2 Test Systems are designed to detect antibodies against both the Nucleocapsid protein and the Spike protein S1-RBD and are optimized for superior sensitivity and specificity in the detection of antibodies in serum and plasma. The design allows for detection of antibodies produced by a natural SARS-CoV-2 infection and/or from vaccination. Are you missing any targets in the SARS-CoV-2 antibody test you use today?
Two antigens are better than one
ZEUS’s dual antigen approach is unique as most available tests are limited to one or the other. By detecting antibodies directed against both the highly immunogenic N-protein and the neutralizing S1-RBD our assay is optimized for superior sensitivity and specificity.
Both the ZEUS ELISA SARS-CoV-2 IgG Test System and ZEUS ELISA SARS-CoV-2 Total (IgG, IgA, IgM) Antibody Test System are authorized by the U.S. Food and Drug Administration (FDA) for Emergency Use Authorization (EUA) as diagnostic tests for the qualitative detection of antibodies to the SARS-CoV-2 virus in human serum and plasma. Both ZEUS ELISA products are in stock and ready to ship today.
 |
See firsthand the performance of utilizing two antigens for both S1-RBD and nucleoprotein. Contact sales@zeusscientific.com for evaluation kits of the ZEUS ELISA SARS-CoV-2 Test Systems. |
The Dual Antigen Difference: Specificity Matters
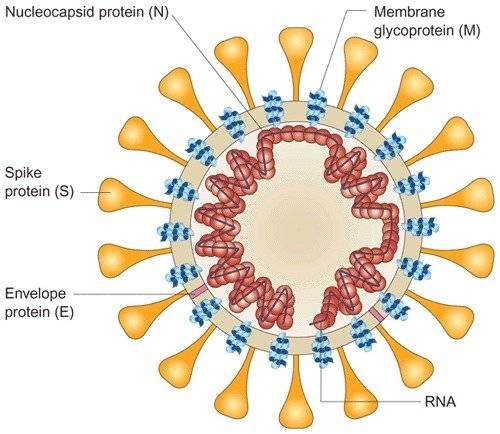 |
S1-RBD: The surface glycoprotein of the virus, termed the spike (S) protein, mediates attachment of the virus to human cells via its receptor‐binding domain (RBD; Wrapp et al., 2020) and mediates fusion of viral and cellular membranes. Antibodies binding to the spike protein, and especially to the RBD domain, can neutralize SARS‐CoV‐2. |
N-Protein: The nucleocapsid protein (N-protein) is the most abundant protein in coronavirus. The N-protein is a highly immunogenic phosphoprotein, and it is normally very conserved. The N protein of coronavirus is often used as a marker in diagnostic assays. During virion assembly, N protein binds to viral RNA and leads to formation of the helical nucleocapsid. The abundance and high hydrophilicity of N protein are supposed to contribute to potent immunity after coronavirus infection.
ZEUS ELISA SARS-CoV-2 Antibody Test Systems
- ZEUS ELISA SARS-CoV-2 IgG Antibody Test System
- ZEUS ELISA SARS-CoV-2 Total Antibody Test System
Both the ZEUS ELISA SARS-CoV-2 Test Systems are intended for use as an aid in identifying individuals with an adaptive immune response to SARS-CoV-2, indicating recent or prior infection. The assay utilizes a dual antigen combination of recombinant S1 receptor binding domain (RBD) viral protein and recombinant nucleoprotein for optimal performance.
ZEUS Universal ELISA Protocol
 |
Both SARS-CoV-2 IgG Test System assays follow ZEUS’s universal ELISA assay protocol, developed to harmonize with your existing test menu and volume needs. Simply put, our SARS-CoV-2 Antibody assays share ZEUS’s common components, incubations performed at room temperature and incubation timings which enable your laboratory to multi-task and benefit with faster, flexible test systems designed to make your day easier. |
Scalable to your laboratory needs!
Both the ZEUS SARS-CoV-2 IgG Test Systems and ZEUS SARS-CoV-2 Total Antibody Test Systems have FDA EUA to run manually or with the Dynex Agility®, DSX® and DS2®. The Dynex Agility® offers high throughput and takes advantage of the SmartKit® Gold packaging, providing the easy ability to fully automate the procedure from sample to result in a throughput meeting all laboratory requirements. These new test systems include our proprietary SAVe Diluent, a unique component which changes color when serum is added ensuring no well is missed!
Wrapp, D., Wang, N., Corbett, K. S., Goldsmith, J. A., Hsieh, C. L., Abiona, O., … McLellan, J. S. (2020). Cryo‐EM structure of the 2019‐nCoV spike in the prefusion conformation. Science, 367(6483), 1260–1263. doi: 10.1126/science.abb2507.
FDA EUA Disclaimer: The ZEUS ELISA SARS-CoV-2 IgG test has been authorized only for the presence of IgG antibodies against SARS-CoV-2, not for any other viruses or pathogens. The ZEUS ELISA SARS-CoV-2 Total Antibody test has been authorized only for the presence of total antibodies against SARS-CoV-2, not for any other viruses or pathogens. These tests have been authorized by FDA under an EUA for use by authorized laboratories. These tests have not been FDA cleared or approved; These tests are only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner.
