Lyme Disease
ZEUS Borrelia MTTT®: A paradigm shift in testing for Lyme disease
ZEUS Scientific has the expertise you deserve. Partner with the pioneering experts in Lyme disease testing since 1987.
 |
Lyme disease, if diagnosed and treated early, can be cured completely, however early cases are often missed by standard lab testing methods - delaying the proper treatment.1
Now there's a better way!
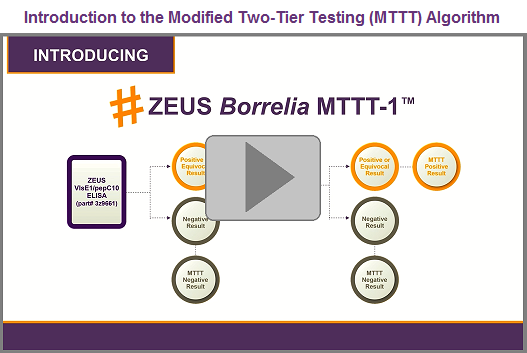
The all-ELISA ZEUS Borrelia Modified Two-Tiered Testing (MTTT) algorithm |

In recent clinical studies, ZEUS Borrelia MTTT demonstrated superior sensitivity vs. the Standard Two-Tiered Testing (STTT) algorithm with comparable specificity.
It’s time for a paradigm shift in Lyme disease testing.
Let's change the game together!
To learn how easy it is to adopt the ZEUS Borrelia MTTT algorithm in your laboratory, e-mail us today!
To speak with an Account Manager about ZEUS Borrelia MTTT or our broad menu of simple and flexible infectious disease and autoimmune disease test systems, please call 1-800-286-2111 or (908) 526-3744.
References:
1 http://professionals.site.apic.org/bugs-and-outbreaks/lyme-disease/
*Data on file at ZEUS Scientific